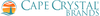Is Phosphoric Acid Bad for You?
SUBSCRIBE TO OUR BLOG
Promotions, new products, and recipes.
Phosphoric acid is commonly used in various industries, including food and beverages, fertilizers, and detergents. But what are the effects of this mineral on your health? In this article, we'll explore common misconceptions about phosphoric acid and provide evidence-based information to help you understand its true impact.
Key Takeaways:
- Phosphoric acid is commonly used in various industries, including food and beverages, fertilizers, and detergents.
- Despite common misconceptions, phosphoric acid does not have a significant negative impact on human health when consumed in moderation.
- Proper handling, storage, and disposal of phosphoric acid are important to minimize potential risks.
- Phosphoric acid has potential benefits for bone health, digestion, and nutrient absorption.
- The environmental impact of phosphoric acid production should be taken into consideration, and sustainable practices should be implemented where possible.
Understanding Phosphoric Acid
Phosphoric acid is an inorganic acid that is commonly used in various industries. It is a clear, colorless liquid that is soluble in water and has a high boiling point. The properties of phosphoric acid depend on its concentration and temperature.
Phosphoric acid is a triprotic acid, which means that it can dissolve three hydrogen ions in water. Its chemical formula is H3PO4, and it has a molecular weight of 97.99 g/mol.
The production of phosphoric acid involves the reaction between phosphate rock and sulfuric acid. The byproduct of this reaction is commonly called "wet process" phosphoric acid. Another method of producing phosphoric acid is the thermal process, which involves melting phosphate rock with coke and silica. This process produces "thermal process" phosphoric acid.
| Properties of Phosphoric Acid | Production Methods |
|---|---|
|
|
Phosphoric acid is commonly used in the manufacturing of fertilizers, detergents, and food additives. It is also used in the production of steel, cleaning agents, and other goods. Its versatility and usefulness make it an essential chemical in many industries.
In the next section, we will explore the use of phosphoric acid in food and beverages, and its potential health implications.
Phosphoric Acid in Food and Beverages
Phosphoric acid is commonly used as a food additive in various food and beverages, including carbonated soft drinks, processed meats, and cheese. It acts as a preservative, acidulant, and flavoring agent, enhancing the taste and texture of these products. However, the consumption of phosphoric acid in large amounts can have potential health implications.
| Products | Phosphoric Acid Content (mg/serving) |
|---|---|
| Cola drinks | Approximately 20-70 mg/100 ml |
| Processed meats | 15-70 mg/100 g |
| Cheese | 450-650 mg/100 g |
While small amounts of phosphoric acid are generally considered safe for human consumption, excessive intake can lead to adverse effects. It can interfere with the body's calcium absorption and increase the risk of bone loss, particularly in people who have calcium deficiencies or consume low-calcium diets. Furthermore, frequent consumption of phosphoric acid-containing drinks has been linked to dental erosion and cavities.
Therefore, it is important to consume foods and beverages with phosphoric acid in moderation and balance them with calcium-rich foods to prevent any potential negative health outcomes. Choosing natural, unprocessed foods without added phosphoric acid can also be beneficial for overall health.
Industrial Applications of Phosphoric Acid
Phosphoric acid is a crucial component in various industrial processes, making it an irreplaceable substance in several fields. Its versatility and compatibility with a wide range of other chemicals make it a go-to option for many manufacturers. Let's take a closer look at some of the industries that rely on phosphoric acid:
Metallurgy
Phosphoric acid is an essential component of stainless steel production, as it is used to remove impurities and increase the overall quality of the metal. It is also used in other metallurgical applications, such as the production of aluminum alloys and the refining of rare earth metals.
Fertilizers
Phosphoric acid is a primary ingredient in various fertilizers, as it is essential for plant growth and development. It is used to create phosphate fertilizers that provide essential nutrients to crops and promote healthy soil conditions.
Detergents
Disodium phosphate, a compound derived from phosphoric acid, is commonly used in cleaning products such as dishwashing detergents. Its ability to soften water and remove grime and grease makes it an ideal ingredient in various cleaning formulations.
Image related to Industrial Applications of Phosphoric Acid

Other Applications
Aside from the industries mentioned above, phosphoric acid is also used in the production of synthetic resins, food additives, and flame retardants. Its ability to act as both an acid and a chelating agent makes it an adaptable and widely-used substance.
Phosphoric acid is a vital component in several industrial processes, thanks to its unique properties and versatility. Its importance in various industries will undoubtedly lead to its continued use for years to come.
The Production of Phosphoric Acid
Phosphoric acid, also known as orthophosphoric acid, is a colorless and odorless inorganic acid that is widely used in industries such as food, agriculture, and pharmaceuticals. It is primarily produced by two methods – the thermal process and the wet process.
The Thermal Process
In the thermal process, phosphate rock is burned at high temperatures, resulting in the formation of phosphoric acid and a byproduct called “gypsum” which is a calcium sulfate compound. This process requires a high amount of energy and produces a considerable amount of greenhouse gases, making it less environmentally friendly.
The Wet Process
The wet process is a more commonly used technique for producing phosphoric acid. In this process, phosphate rock is treated with sulfuric acid to form phosphoric acid and gypsum as a byproduct. The phosphoric acid is then purified through a series of steps to obtain the desired concentration for various applications.
The wet process produces less waste and requires less energy compared to the thermal process, making it a more sustainable option for phosphoric acid production.
Environmental Impacts
Phosphoric acid production, particularly through the thermal process, poses various environmental risks. The high-energy consumption and emissions generated during the process contribute to air pollution and greenhouse gas emissions, while the discharge of waste byproducts into surrounding ecosystems can lead to soil and water pollution.
To mitigate its environmental impact, sustainable practices such as the use of renewable energy sources and closed-loop systems for waste management should be incorporated into phosphoric acid production processes.
Benefits of Phosphoric Acid
While there are concerns about the impact of phosphoric acid on health, it also offers potential benefits.
- Bone Health: Phosphoric acid is a key component of bone tissue, playing a crucial role in maintaining bone density and strength. Studies suggest that the consumption of phosphoric acid may help reduce the risk of osteoporosis and bone fractures.
- Digestion: Phosphoric acid is commonly used in digestive aids and antacids, owing to its ability to neutralize stomach acid. It can also enhance digestion by breaking down proteins more effectively.
- Nutrient Absorption: Phosphoric acid can improve the absorption of essential nutrients such as calcium, magnesium, and iron, providing numerous health benefits.
Of course, it is important to consult with a healthcare professional before consuming phosphoric acid to reap these potential benefits. Additionally, it is essential to ensure that you are consuming it in safe and appropriate quantities.
Safety Precautions for Phosphoric Acid
Phosphoric acid is a powerful and potentially hazardous substance that requires proper handling and usage to prevent harm. Here are some essential safety precautions to keep in mind when working with phosphoric acid:
| Precautions | Explanation |
|---|---|
| Wear Protective Clothing | When handling phosphoric acid, always wear protective clothing such as gloves, safety goggles, and a lab coat to protect your skin and eyes from burns or damage. |
| Handle with Care | Phosphoric acid is a strong acid that can cause severe burns or injuries if it comes into contact with the skin or eyes. Handle it carefully and avoid any direct contact with the substance. |
| Avoid Inhalation | Phosphoric acid vapors can be harmful to your respiratory system. Always work in a well-ventilated area and wear a respirator mask if necessary. |
| Store Properly | Store phosphoric acid in a cool and dry place, away from sources of heat or sunlight. Keep it in a properly labeled and securely closed container to prevent accidental spills or leaks. |
| Dispose Safely | Phosphoric acid must be disposed of safely according to local regulations. Don't pour it down the drain or discard it in the trash. Follow proper disposal guidelines to avoid harming the environment or risking exposure to others. |
These safety precautions can help prevent accidents and ensure that phosphoric acid is used safely and responsibly. By following these guidelines, you can minimize the risks associated with this powerful substance and protect yourself and others from harm.

Common Misconceptions about Phosphoric Acid
Phosphoric acid has been the subject of numerous misconceptions over the years, often causing confusion and concern. Contrary to popular belief, phosphoric acid does not have a negative impact on bone health or tooth enamel when consumed in moderate amounts. In fact, this acid is an important component in many food and beverage products, including soft drinks.
One common misconception is that phosphoric acid causes osteoporosis, a medical condition characterized by weak and brittle bones. However, studies have shown that moderate consumption of phosphoric acid does not affect bone density or increase the risk of osteoporosis. Additionally, claims that the acid causes tooth decay or erosion are unfounded. The acid is widely used as a flavoring and preservative in various foods and beverages and is considered safe for consumption in moderate amounts.
Ultimately, the misinformation surrounding phosphoric acid can be misleading. As with any ingredient, it is essential to understand its properties, effects, and safe usage to make informed decisions. By dispelling these common misconceptions, we can appreciate the multiple benefits of phosphoric acid in our daily lives.
Usage of Phosphoric Acid in Everyday Life
Phosphoric acid is a versatile compound with numerous everyday uses, making it an essential ingredient in many household products. Its ability to adjust pH levels, remove rust, and act as a cleaning agent makes it indispensable in various applications.
As a pH Adjuster:
Phosphoric acid is commonly used as a pH adjuster in many industries, including food and agriculture. It is added to food products such as cola and processed cheese to provide a tangy flavor and enhance preservation. In agriculture, it is used to adjust the acidity of the soil, making it more suitable for crop growth.
As a Rust Remover:
Phosphoric acid is a powerful agent for removing rust and other metal oxides. It is used extensively in the automotive industry as a rust remover and as a pre-paint treatment for metal surfaces. It can also be found in household cleaning products such as Lime-A-Way, a popular descaling cleaner for bathrooms and kitchens.
As a Cleaning Agent:
Phosphoric acid's acidic nature makes it an excellent cleaning agent for many surfaces, particularly those with hard water stains or mineral build-up. It is used in various cleaning products, including CLR, a household cleaner used for removing calcium, lime, and rust stains. Phosphoric acid is also found in some dishwasher detergents used to clean dishes and remove hard water stains.
"Phosphoric acid's ability to adjust pH levels, remove rust, and act as a cleaner makes it indispensable in various applications."
Environmental Impact of Phosphoric Acid Production
While phosphoric acid has various industrial and household uses, its production can have negative impacts on the environment. The production process requires significant energy consumption, which can contribute to greenhouse gas emissions and climate change. Additionally, the extraction of phosphate rock, the primary raw material used in production, can lead to soil degradation and water pollution.
Furthermore, the waste generated during the production process can contain hazardous substances that must be properly managed to prevent environmental damage. Inadequate waste management can lead to contamination of water sources and harm to wildlife and ecosystems.
Efforts are being made to reduce the environmental impact of phosphoric acid production. For example, some production facilities are implementing sustainable practices such as using renewable energy sources, optimizing water usage, and reducing waste generation.
As consumers, we can also play a role in reducing the environmental impact of phosphoric acid production. By choosing products made using sustainable production methods and properly disposing of waste, we can contribute to a cleaner, healthier environment.
Safe Usage of Phosphoric Acid in Food and Industry
Phosphoric acid is commonly used in both food and industrial settings. However, it is essential to follow safety guidelines to minimize any potential risks. Here are some recommendations:
Food
When using phosphoric acid in food production:
- Make sure to properly label and store containers to avoid accidental ingestion.
- Follow the recommended dosages and avoid excess usage to prevent over-acidification.
- Consider using alternatives to phosphoric acid in products intended for children or those with sensitivities or medical conditions.
- Ensure proper ventilation during the mixing and application of phosphoric acid to prevent inhalation of fumes.
- Avoid skin or eye contact by wearing protective gear such as gloves and goggles.
Industry
In industrial applications:
- Provide worker training on safe handling procedures, the appropriate use of PPE, and emergency responses in case of accidents or spills.
- Store phosphoric acid containers in a dedicated area, away from other chemicals that could react with it or cause cross-contamination.
- Implement measures to prevent spills or leaks, such as secondary containment systems or regular inspections of pipes and valves.
- Dispose of phosphoric acid waste in compliance with local and federal regulations, avoiding any harmful effects on the environment or public health.
By following these guidelines, phosphoric acid can be used safely and effectively in both food and industrial settings.
Conclusion
In conclusion, the article delved into the topic of phosphoric acid, addressing common misconceptions and providing an in-depth overview of its properties, production, and usage. While some concerns have been raised regarding its potential impact on health and the environment, research indicates that when used appropriately, phosphoric acid can provide important benefits and functions in a variety of applications.
It is important to follow safety precautions and guidelines for proper handling, storage, and disposal, particularly in food and industrial settings. By taking these precautions, minimal risks can be ensured.
In summary, phosphoric acid is an essential component of modern manufacturing and everyday life. Its versatility and importance cannot be understated, and continued research and development will help to optimize its usage while minimizing any potential negative effects.
FAQ
Is phosphoric acid bad for you?
Despite common misconceptions, phosphoric acid is generally considered safe for consumption in moderate amounts. However, excessive intake can have negative health effects, particularly on dental health and bone mineralization.
What are the properties of phosphoric acid?
Phosphoric acid is a clear, odorless liquid with a sour taste. It is highly soluble in water and has a relatively high boiling point. It is also corrosive and can react with metals and organic compounds.
How is phosphoric acid produced?
Phosphoric acid is primarily produced through the wet process, which involves treating phosphate ore with sulfuric acid. This process allows for the extraction of phosphoric acid in various concentrations.
Where is phosphoric acid commonly found in food and beverages?
Phosphoric acid is commonly found in carbonated soft drinks, where it is used as a flavoring agent and preservative. It is also present in processed meats, cheeses, and some baked goods, acting as an acidulant and enhancing flavor.
What are the industrial applications of phosphoric acid?
Phosphoric acid finds extensive use in various industries. It is utilized in the production of fertilizers, detergents, pharmaceuticals, metal treatment, and water treatment processes, as well as in the synthesis of other chemicals.
What are the benefits of phosphoric acid?
Phosphoric acid has several benefits, including aiding in digestion, promoting the absorption of nutrients, and playing a crucial role in maintaining bone health. It is also used in dental care products to help prevent the formation of dental plaque.
What safety precautions should be taken when handling phosphoric acid?
When handling phosphoric acid, it is important to wear protective clothing, gloves, and goggles to prevent skin and eye contact. It should be stored in a cool, well-ventilated area, away from incompatible materials, and handled with care to avoid spills or exposure.
What are some common misconceptions about phosphoric acid?
Some common misconceptions about phosphoric acid include its alleged negative impact on tooth enamel and bone health. However, scientific studies have shown that moderate consumption of phosphoric acid in food and beverages is unlikely to cause significant harm.
What are the everyday uses of phosphoric acid?
Phosphoric acid has a wide range of everyday uses. It is used as a pH adjuster in some food and beverage products, a rust remover, a cleaning agent for household items, and as an ingredient in certain cosmetics and personal care products.
What is the environmental impact of phosphoric acid production?
The production of phosphoric acid can have significant environmental impacts. It consumes large amounts of energy and water, and the disposal of phosphogypsum, a byproduct of the production process, can pose challenges due to its potential impact on soil and water quality.
How can phosphoric acid be safely used in food and industry?
To ensure safe usage of phosphoric acid in food and industrial settings, it is important to follow proper handling procedures, including wearing appropriate protective equipment and adhering to recommended concentration levels. Proper storage and disposal of phosphoric acid are also crucial.
Phosphoric acid is commonly used in various industries, including food and beverages, fertilizers, and detergents. But what are the effects of this mineral on your health? In this article, we'll explore common misconceptions about phosphoric acid and provide evidence-based information to help you understand its true impact.
Key Takeaways:
- Phosphoric acid is commonly used in various industries, including food and beverages, fertilizers, and detergents.
- Despite common misconceptions, phosphoric acid does not have a significant negative impact on human health when consumed in moderation.
- Proper handling, storage, and disposal of phosphoric acid are important to minimize potential risks.
- Phosphoric acid has potential benefits for bone health, digestion, and nutrient absorption.
- The environmental impact of phosphoric acid production should be taken into consideration, and sustainable practices should be implemented where possible.
Understanding Phosphoric Acid
Phosphoric acid is an inorganic acid that is commonly used in various industries. It is a clear, colorless liquid that is soluble in water and has a high boiling point. The properties of phosphoric acid depend on its concentration and temperature.
Phosphoric acid is a triprotic acid, which means that it can dissolve three hydrogen ions in water. Its chemical formula is H3PO4, and it has a molecular weight of 97.99 g/mol.
The production of phosphoric acid involves the reaction between phosphate rock and sulfuric acid. The byproduct of this reaction is commonly called "wet process" phosphoric acid. Another method of producing phosphoric acid is the thermal process, which involves melting phosphate rock with coke and silica. This process produces "thermal process" phosphoric acid.
| Properties of Phosphoric Acid | Production Methods |
|---|---|
|
|
Phosphoric acid is commonly used in the manufacturing of fertilizers, detergents, and food additives. It is also used in the production of steel, cleaning agents, and other goods. Its versatility and usefulness make it an essential chemical in many industries.
In the next section, we will explore the use of phosphoric acid in food and beverages, and its potential health implications.
Phosphoric Acid in Food and Beverages
Phosphoric acid is commonly used as a food additive in various food and beverages, including carbonated soft drinks, processed meats, and cheese. It acts as a preservative, acidulant, and flavoring agent, enhancing the taste and texture of these products. However, the consumption of phosphoric acid in large amounts can have potential health implications.
| Products | Phosphoric Acid Content (mg/serving) |
|---|---|
| Cola drinks | Approximately 20-70 mg/100 ml |
| Processed meats | 15-70 mg/100 g |
| Cheese | 450-650 mg/100 g |
While small amounts of phosphoric acid are generally considered safe for human consumption, excessive intake can lead to adverse effects. It can interfere with the body's calcium absorption and increase the risk of bone loss, particularly in people who have calcium deficiencies or consume low-calcium diets. Furthermore, frequent consumption of phosphoric acid-containing drinks has been linked to dental erosion and cavities.
Therefore, it is important to consume foods and beverages with phosphoric acid in moderation and balance them with calcium-rich foods to prevent any potential negative health outcomes. Choosing natural, unprocessed foods without added phosphoric acid can also be beneficial for overall health.
Industrial Applications of Phosphoric Acid
Phosphoric acid is a crucial component in various industrial processes, making it an irreplaceable substance in several fields. Its versatility and compatibility with a wide range of other chemicals make it a go-to option for many manufacturers. Let's take a closer look at some of the industries that rely on phosphoric acid:
Metallurgy
Phosphoric acid is an essential component of stainless steel production, as it is used to remove impurities and increase the overall quality of the metal. It is also used in other metallurgical applications, such as the production of aluminum alloys and the refining of rare earth metals.
Fertilizers
Phosphoric acid is a primary ingredient in various fertilizers, as it is essential for plant growth and development. It is used to create phosphate fertilizers that provide essential nutrients to crops and promote healthy soil conditions.
Detergents
Disodium phosphate, a compound derived from phosphoric acid, is commonly used in cleaning products such as dishwashing detergents. Its ability to soften water and remove grime and grease makes it an ideal ingredient in various cleaning formulations.
Image related to Industrial Applications of Phosphoric Acid

Other Applications
Aside from the industries mentioned above, phosphoric acid is also used in the production of synthetic resins, food additives, and flame retardants. Its ability to act as both an acid and a chelating agent makes it an adaptable and widely-used substance.
Phosphoric acid is a vital component in several industrial processes, thanks to its unique properties and versatility. Its importance in various industries will undoubtedly lead to its continued use for years to come.
The Production of Phosphoric Acid
Phosphoric acid, also known as orthophosphoric acid, is a colorless and odorless inorganic acid that is widely used in industries such as food, agriculture, and pharmaceuticals. It is primarily produced by two methods – the thermal process and the wet process.
The Thermal Process
In the thermal process, phosphate rock is burned at high temperatures, resulting in the formation of phosphoric acid and a byproduct called “gypsum” which is a calcium sulfate compound. This process requires a high amount of energy and produces a considerable amount of greenhouse gases, making it less environmentally friendly.
The Wet Process
The wet process is a more commonly used technique for producing phosphoric acid. In this process, phosphate rock is treated with sulfuric acid to form phosphoric acid and gypsum as a byproduct. The phosphoric acid is then purified through a series of steps to obtain the desired concentration for various applications.
The wet process produces less waste and requires less energy compared to the thermal process, making it a more sustainable option for phosphoric acid production.
Environmental Impacts
Phosphoric acid production, particularly through the thermal process, poses various environmental risks. The high-energy consumption and emissions generated during the process contribute to air pollution and greenhouse gas emissions, while the discharge of waste byproducts into surrounding ecosystems can lead to soil and water pollution.
To mitigate its environmental impact, sustainable practices such as the use of renewable energy sources and closed-loop systems for waste management should be incorporated into phosphoric acid production processes.
Benefits of Phosphoric Acid
While there are concerns about the impact of phosphoric acid on health, it also offers potential benefits.
- Bone Health: Phosphoric acid is a key component of bone tissue, playing a crucial role in maintaining bone density and strength. Studies suggest that the consumption of phosphoric acid may help reduce the risk of osteoporosis and bone fractures.
- Digestion: Phosphoric acid is commonly used in digestive aids and antacids, owing to its ability to neutralize stomach acid. It can also enhance digestion by breaking down proteins more effectively.
- Nutrient Absorption: Phosphoric acid can improve the absorption of essential nutrients such as calcium, magnesium, and iron, providing numerous health benefits.
Of course, it is important to consult with a healthcare professional before consuming phosphoric acid to reap these potential benefits. Additionally, it is essential to ensure that you are consuming it in safe and appropriate quantities.
Safety Precautions for Phosphoric Acid
Phosphoric acid is a powerful and potentially hazardous substance that requires proper handling and usage to prevent harm. Here are some essential safety precautions to keep in mind when working with phosphoric acid:
| Precautions | Explanation |
|---|---|
| Wear Protective Clothing | When handling phosphoric acid, always wear protective clothing such as gloves, safety goggles, and a lab coat to protect your skin and eyes from burns or damage. |
| Handle with Care | Phosphoric acid is a strong acid that can cause severe burns or injuries if it comes into contact with the skin or eyes. Handle it carefully and avoid any direct contact with the substance. |
| Avoid Inhalation | Phosphoric acid vapors can be harmful to your respiratory system. Always work in a well-ventilated area and wear a respirator mask if necessary. |
| Store Properly | Store phosphoric acid in a cool and dry place, away from sources of heat or sunlight. Keep it in a properly labeled and securely closed container to prevent accidental spills or leaks. |
| Dispose Safely | Phosphoric acid must be disposed of safely according to local regulations. Don't pour it down the drain or discard it in the trash. Follow proper disposal guidelines to avoid harming the environment or risking exposure to others. |
These safety precautions can help prevent accidents and ensure that phosphoric acid is used safely and responsibly. By following these guidelines, you can minimize the risks associated with this powerful substance and protect yourself and others from harm.

Common Misconceptions about Phosphoric Acid
Phosphoric acid has been the subject of numerous misconceptions over the years, often causing confusion and concern. Contrary to popular belief, phosphoric acid does not have a negative impact on bone health or tooth enamel when consumed in moderate amounts. In fact, this acid is an important component in many food and beverage products, including soft drinks.
One common misconception is that phosphoric acid causes osteoporosis, a medical condition characterized by weak and brittle bones. However, studies have shown that moderate consumption of phosphoric acid does not affect bone density or increase the risk of osteoporosis. Additionally, claims that the acid causes tooth decay or erosion are unfounded. The acid is widely used as a flavoring and preservative in various foods and beverages and is considered safe for consumption in moderate amounts.
Ultimately, the misinformation surrounding phosphoric acid can be misleading. As with any ingredient, it is essential to understand its properties, effects, and safe usage to make informed decisions. By dispelling these common misconceptions, we can appreciate the multiple benefits of phosphoric acid in our daily lives.
Usage of Phosphoric Acid in Everyday Life
Phosphoric acid is a versatile compound with numerous everyday uses, making it an essential ingredient in many household products. Its ability to adjust pH levels, remove rust, and act as a cleaning agent makes it indispensable in various applications.
As a pH Adjuster:
Phosphoric acid is commonly used as a pH adjuster in many industries, including food and agriculture. It is added to food products such as cola and processed cheese to provide a tangy flavor and enhance preservation. In agriculture, it is used to adjust the acidity of the soil, making it more suitable for crop growth.
As a Rust Remover:
Phosphoric acid is a powerful agent for removing rust and other metal oxides. It is used extensively in the automotive industry as a rust remover and as a pre-paint treatment for metal surfaces. It can also be found in household cleaning products such as Lime-A-Way, a popular descaling cleaner for bathrooms and kitchens.
As a Cleaning Agent:
Phosphoric acid's acidic nature makes it an excellent cleaning agent for many surfaces, particularly those with hard water stains or mineral build-up. It is used in various cleaning products, including CLR, a household cleaner used for removing calcium, lime, and rust stains. Phosphoric acid is also found in some dishwasher detergents used to clean dishes and remove hard water stains.
"Phosphoric acid's ability to adjust pH levels, remove rust, and act as a cleaner makes it indispensable in various applications."
Environmental Impact of Phosphoric Acid Production
While phosphoric acid has various industrial and household uses, its production can have negative impacts on the environment. The production process requires significant energy consumption, which can contribute to greenhouse gas emissions and climate change. Additionally, the extraction of phosphate rock, the primary raw material used in production, can lead to soil degradation and water pollution.
Furthermore, the waste generated during the production process can contain hazardous substances that must be properly managed to prevent environmental damage. Inadequate waste management can lead to contamination of water sources and harm to wildlife and ecosystems.
Efforts are being made to reduce the environmental impact of phosphoric acid production. For example, some production facilities are implementing sustainable practices such as using renewable energy sources, optimizing water usage, and reducing waste generation.
As consumers, we can also play a role in reducing the environmental impact of phosphoric acid production. By choosing products made using sustainable production methods and properly disposing of waste, we can contribute to a cleaner, healthier environment.
Safe Usage of Phosphoric Acid in Food and Industry
Phosphoric acid is commonly used in both food and industrial settings. However, it is essential to follow safety guidelines to minimize any potential risks. Here are some recommendations:
Food
When using phosphoric acid in food production:
- Make sure to properly label and store containers to avoid accidental ingestion.
- Follow the recommended dosages and avoid excess usage to prevent over-acidification.
- Consider using alternatives to phosphoric acid in products intended for children or those with sensitivities or medical conditions.
- Ensure proper ventilation during the mixing and application of phosphoric acid to prevent inhalation of fumes.
- Avoid skin or eye contact by wearing protective gear such as gloves and goggles.
Industry
In industrial applications:
- Provide worker training on safe handling procedures, the appropriate use of PPE, and emergency responses in case of accidents or spills.
- Store phosphoric acid containers in a dedicated area, away from other chemicals that could react with it or cause cross-contamination.
- Implement measures to prevent spills or leaks, such as secondary containment systems or regular inspections of pipes and valves.
- Dispose of phosphoric acid waste in compliance with local and federal regulations, avoiding any harmful effects on the environment or public health.
By following these guidelines, phosphoric acid can be used safely and effectively in both food and industrial settings.
Conclusion
In conclusion, the article delved into the topic of phosphoric acid, addressing common misconceptions and providing an in-depth overview of its properties, production, and usage. While some concerns have been raised regarding its potential impact on health and the environment, research indicates that when used appropriately, phosphoric acid can provide important benefits and functions in a variety of applications.
It is important to follow safety precautions and guidelines for proper handling, storage, and disposal, particularly in food and industrial settings. By taking these precautions, minimal risks can be ensured.
In summary, phosphoric acid is an essential component of modern manufacturing and everyday life. Its versatility and importance cannot be understated, and continued research and development will help to optimize its usage while minimizing any potential negative effects.
FAQ
Is phosphoric acid bad for you?
Despite common misconceptions, phosphoric acid is generally considered safe for consumption in moderate amounts. However, excessive intake can have negative health effects, particularly on dental health and bone mineralization.
What are the properties of phosphoric acid?
Phosphoric acid is a clear, odorless liquid with a sour taste. It is highly soluble in water and has a relatively high boiling point. It is also corrosive and can react with metals and organic compounds.
How is phosphoric acid produced?
Phosphoric acid is primarily produced through the wet process, which involves treating phosphate ore with sulfuric acid. This process allows for the extraction of phosphoric acid in various concentrations.
Where is phosphoric acid commonly found in food and beverages?
Phosphoric acid is commonly found in carbonated soft drinks, where it is used as a flavoring agent and preservative. It is also present in processed meats, cheeses, and some baked goods, acting as an acidulant and enhancing flavor.
What are the industrial applications of phosphoric acid?
Phosphoric acid finds extensive use in various industries. It is utilized in the production of fertilizers, detergents, pharmaceuticals, metal treatment, and water treatment processes, as well as in the synthesis of other chemicals.
What are the benefits of phosphoric acid?
Phosphoric acid has several benefits, including aiding in digestion, promoting the absorption of nutrients, and playing a crucial role in maintaining bone health. It is also used in dental care products to help prevent the formation of dental plaque.
What safety precautions should be taken when handling phosphoric acid?
When handling phosphoric acid, it is important to wear protective clothing, gloves, and goggles to prevent skin and eye contact. It should be stored in a cool, well-ventilated area, away from incompatible materials, and handled with care to avoid spills or exposure.
What are some common misconceptions about phosphoric acid?
Some common misconceptions about phosphoric acid include its alleged negative impact on tooth enamel and bone health. However, scientific studies have shown that moderate consumption of phosphoric acid in food and beverages is unlikely to cause significant harm.
What are the everyday uses of phosphoric acid?
Phosphoric acid has a wide range of everyday uses. It is used as a pH adjuster in some food and beverage products, a rust remover, a cleaning agent for household items, and as an ingredient in certain cosmetics and personal care products.
What is the environmental impact of phosphoric acid production?
The production of phosphoric acid can have significant environmental impacts. It consumes large amounts of energy and water, and the disposal of phosphogypsum, a byproduct of the production process, can pose challenges due to its potential impact on soil and water quality.
How can phosphoric acid be safely used in food and industry?
To ensure safe usage of phosphoric acid in food and industrial settings, it is important to follow proper handling procedures, including wearing appropriate protective equipment and adhering to recommended concentration levels. Proper storage and disposal of phosphoric acid are also crucial.

|
About the Author Ed is the founder of Cape Crystal Brands, editor of the Beginner’s Guide to Hydrocolloids, and a passionate advocate for making food science accessible to all. Discover premium ingredients, expert resources, and free formulation tools at capecrystalbrands.com/tools. — Ed |
- Choosing a selection results in a full page refresh.